idk what to go into in uni next year Deadlines for early admission applications are approaching and I'm so overwhelmed. I like math and bio and physics the most in school so I was thinking of going into biomedical engineering, but I'm also interested in genetic engineering and even just straight up bio or physics. I want to innovate AND do research and if possible be involved in creating a new era of outer space exploration. I have all these things that I am interested in and want to do and to have to narrow it down to like...1 or 2 seems so daunting. I'm fascinated by the really big and really small things in physics (and thus comes my passion for outer space, and wanting to know more about this strange thing called our universe) and part
just died cuz I wrote my first 12th grade physics test today Unit test was out of 32 and everything went good until the 24th question and things got really hard and I panicked and sat there unable to add 1+1 for the remaining half hour of the test fuhhhh there goes my mark #theysaidgrade12wouldbeeasierthan11buttheylied
Should I write the Sat Hey I'm in Canada and going into 12th grade this year and I'm wondering if you guys think I should write the SAT. I get really good marks in school and I'm considering a career in sciences or engineering. Discuss.
My last screen is really outdated, so I decided to post this update. I don't play often anymore (better things to do, lack of interest, etc.) but I've had some down time over the past few weeks which lead me to check things out on Maple. It turns out
What effect did Nelson Mandela have on North America For school. I know a bit about what he did in Africa, but how did his actions affect North American life? Or life around the world for that matter? What is the most important part of what he did? Discuss
Issues getting up in the morning Hey Basil. Ever since I entered high school 2 years ago I've been having a horrible time getting up in the morning. I usually set my alarm for 7:10 and leave the house for school by 8:10. I HAVE A PROBLEM THOUGH. I have a horrible time getting out of bed. It takes me a solid 20-30 minutes to actually...well...wake up. I'm always just totally out of it and I have no idea that my alarm will have gone off for 20 minutes by the time I wake up. Once I am awake and aware and all that then it goes fine but just to reach that state of mind is a daily struggle for me. I'm not even up that late compared to other people I know. I'll be up until like 11:10 MAX so I get around 7.5 hours of sleep (which is decent...right?
3ds or 3ds Xl I'm looking to buy Discuss.
Can I somehow assign an order to music Ive downloaded? I downloaded a bunch of songs and I want to make it so that when I put them on my phone they will be recognized and put into an album in order! Any ideas on how I can do this? I'm on windows btw. I thought there'd be an option under properties to assign an order to the songs but it doesn't seem like there is...
Is our Dna programmed to kill us? Are we genetically predetermined to die? I was getting curious and I know that we usually die because of organ failure and illness but...why do we die? Not why as in the philosophical reason, but why as in the biological reason. I heard that at a certain age our cells just stop regenerating and start dying and stuff but WHY?
Help with solutions in 11th grade Chemistry Sorry if I'm posting this in the wrong section. Okay so I got a take-home test in chem today and I'm stumped on the following question. Determine the mass of hydrogen sulfate required to prepare 475.5-mL of sulfuric acid with a pH of 1.77. (6 marks) I'm just confused on how to write the balanced chemical equation because from what I've learned, HSO4- shouldn't be one of the ions that makes up H2SO4, rather it should be 2H+ and SO4-. Anyways, what I have so far is this dissociation equation: H2SO4(aq) ----> H+(aq) + HSO4-(aq) Is this the right place to start? I went ahead and calculated the concentration of H+ but now when I write out my stoichiometric equation I don't know where to put the give
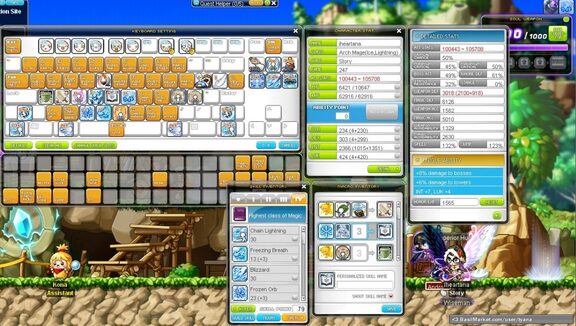
What do you recommend I do with 1.6b to increase my damage? I've recently sold some items and have made my way up to 1.6b. I'm a level 200 I/L mage and am looking for advice on how to use the mesos to best benefit my range! [url=http://imgur.com/a/8DuuO]Equips and stats (imgur album)[/url] If you'd rather not check out the album, my clean range and stats are: a range of 37414-49152, 445m.atk, and 2310 INT. I have 60%INT from equips, and 3%m.atk. Also, I have Kanna link skill Lv. 2, Xenon link skill Lv. 1 (working on 2) and I'm also working on getting the DA link skill. My character card sets add 8% of my mp into my damage range, give me something link +7m.atk, and +40 INT. Thanks for any suggestions! :D

So I figured that it might be fun to do this. Basically my keyboard is set up so that I can do a variety of different things without changing my hand position (so as to get confused). It was FAR better before hypers came out...that messed me up, PLUS
Gms Ice Lightning Hyper Skills When do you think Ice Lightnings in GMS get Hyper Skills? When do you think explorer classes in general get Hypers? So far, I have been out of luck finding dates. I'm so excited though! It will be awesome!